Guess what? Results from a recently published cross-sectional study in Journal of General Internal Medicine reinforce the notion that, despite rigorous efforts to distance medical students and residents from drug and device marketers, these young professionals continue to interface with reps and (wait for it…) they assign increased value to those interactions as their training progresses! (Click here to review the full article.)
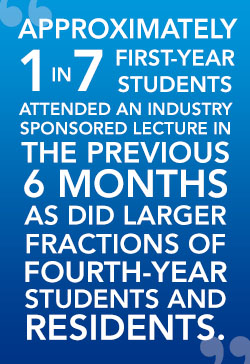
A total of 5,310 first-year medical students, fourth-year medical students, and residents were invited to participate in a survey that measured attitudes towards the perceived influence of industry on their education and their individual participation in industry-sponsored educational initiatives. When 44% responded, that data was stratified according to each institution’s posted limitations towards pharma-trainee interactions (based on the American Medical Student Association”s [AMSA] PharmFree scorecard), and how much federal funding the institution received. In theory, those training at institutions with a higher PharmFree score and those receiving generous government research grants might be more likely to disdain pharma interactions. But, it’s not true!
According to the responses, it appears that medical schools and training programs that attempt to separate industry from medical trainees have done a marginal job communicating and enforcing those policies. For example, individuals matriculating at programs with more strict AMSA Grade A or B PharmFree policies were still eating free meals (somewhere) and attending drug rep presentations, while attesting that their programs had created adequate separation!
Similarly, physicians-in-training at medical centers flush with NIH [National Institutes of Health] grants were just as likely as their colleagues at community hospitals to interact with industry but claimed that the interactions would less likely influence their prescribing decisions.

“Success” should not be defined by eliminating the exchange of free ballpoint pens—a practice some trainees claim still persists.
Results from this survey highlight far more serious concerns. Regardless of training institution, most of these future clinicians admitted feeling unprepared to recognize potential conflicts of interest in dealing with industry. Additionally, they believe faculty members do not fully comply with institutional policies.
What can we take away from this research? It’s time to let the pendulum reverse direction. Quit wasting time trying to plug all the holes in the dike. Instead, devote those resources towards a curriculum that informs and educates physicians-in-training. They should be aware of the potential for conflicts of interest and be offered the analytical tools needed to intelligently assimilate information provided by pharmaceutical reps.
Doctor Lloyd is a clinician-researcher with extensive experience in medical education, instructional design, and innovative content development.
REFERENCE: Austad KE, Avorn J, Franklin JM, et al. Changing interactions between physician trainees and the pharmaceutical industry: a national survey. J Gen Int Med2013; published online February 27, 2013.
IMAGE CREDIT: http://www.upenn.edu/gazette/0706/gaz04.html / Graham Roumieu